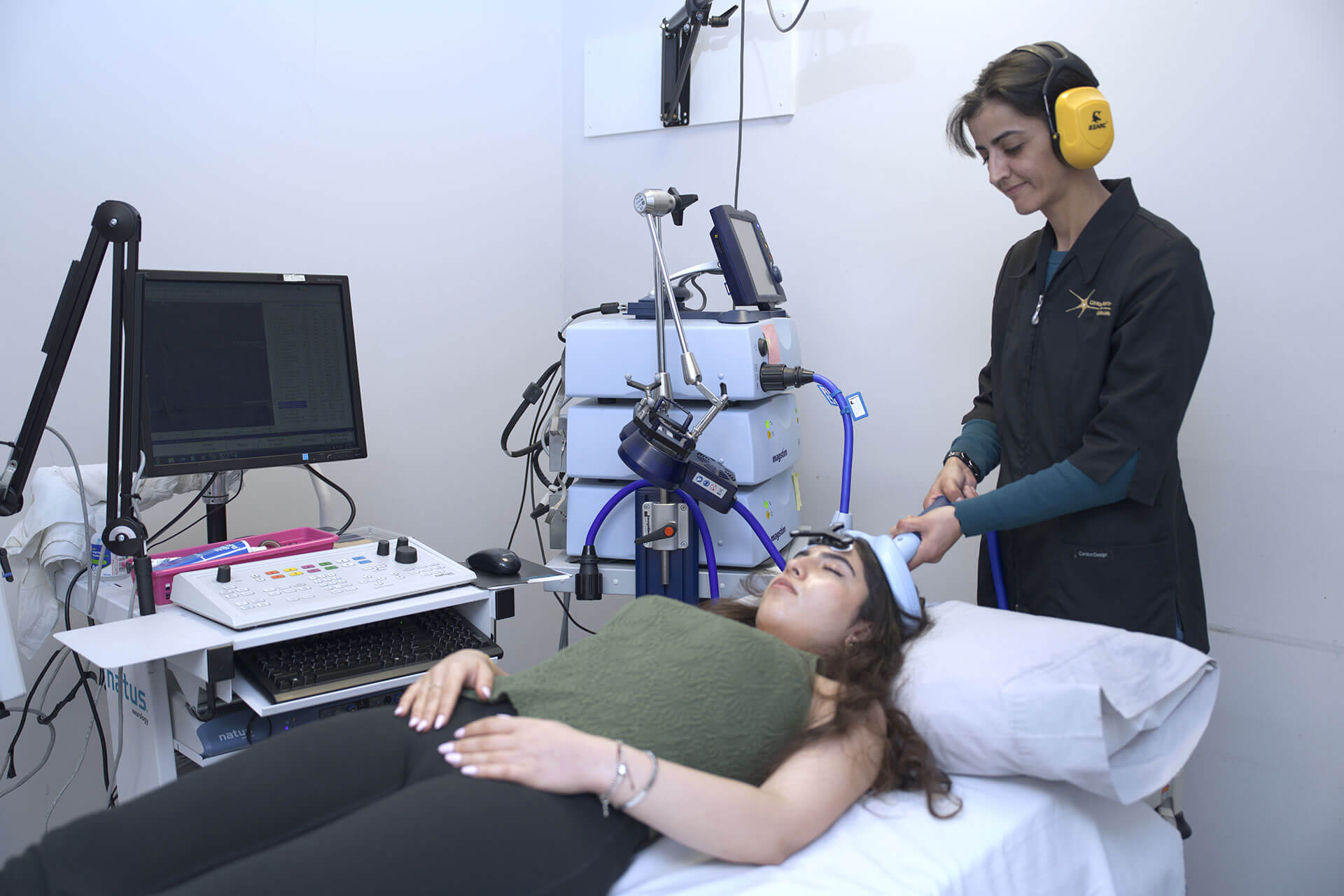
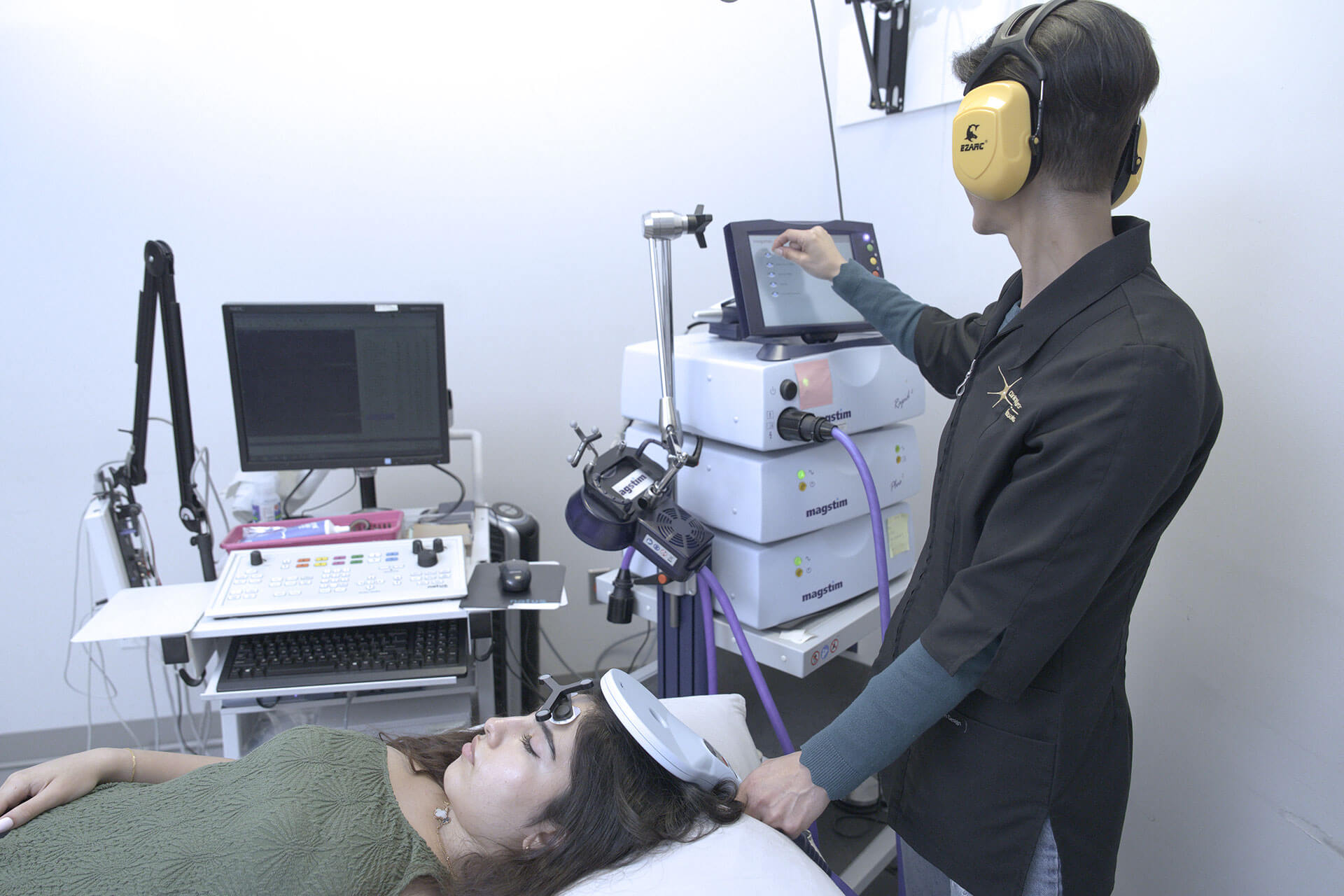
MS symptoms typically begin in the second or third decade of life, but this condition can be diagnosed as early as age 10 and as late as in the 60s. It is a chronic, inflammatory, demyelinating, and neurodegenerative disease of the central nervous system (CNS), believed to result from a genetic predisposition combined with an environmental trigger. The disease process is heterogeneous, with 85% of patients starting with a relapsing-remitting course, which, after 15 to 20 years evolves into a secondary progressive course. The remaining 15% of patients will have a chronic, primary progressive course from the onset.
Common multiple sclerosis symptoms include visual loss, sensory loss in one or both lower extremities, weakness of a limb or loss of balance, walking difficulties.