PML can affect any area of the brain. The clinical presentation in MS patients tend to predominantly implicate cognitive and behavioural symptoms, motor symptoms, visual secondary to optic radiation involvement, speech and seizures. Sensory and gait disturbances are less frequently involved. Optic nerve involvement has yet to be described. Spinal cord on rare occasion has been noted at autopsy. There has yet to be a reported case of clinical myelopathy from PML.
JCV Granule cell Neuronopathy can present with cerebellar signs evolving over months to years accompanied by cerebellar atrophy with or without brainstem hyperintensities.
CSF parameters tend to be normal in the MS PML population. Previous reports of mildly elevated protein and occasional hypoglycorrhachia are probably related to the underlying HIV infection. Detection of the JC virus by PCR can at certain labs detect concentrations as low as 10 copies/ml of CSF. A negative PCR test, however, does not rule out the diagnosis and may need to be repeated. A positive test in the absence of radiological and clinical findings does not confirm the diagnosis as there has been false positive results presumably through CSF-blood contamination.
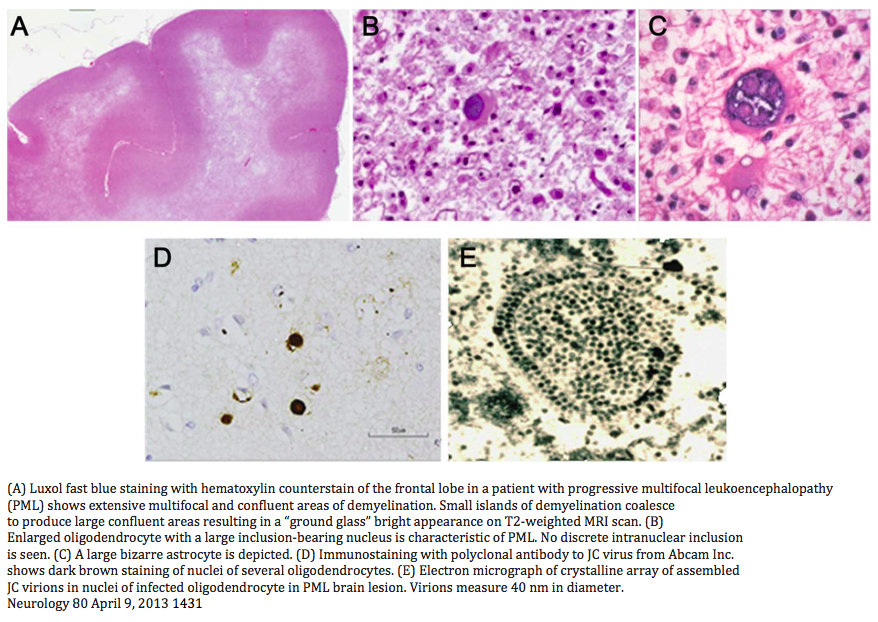
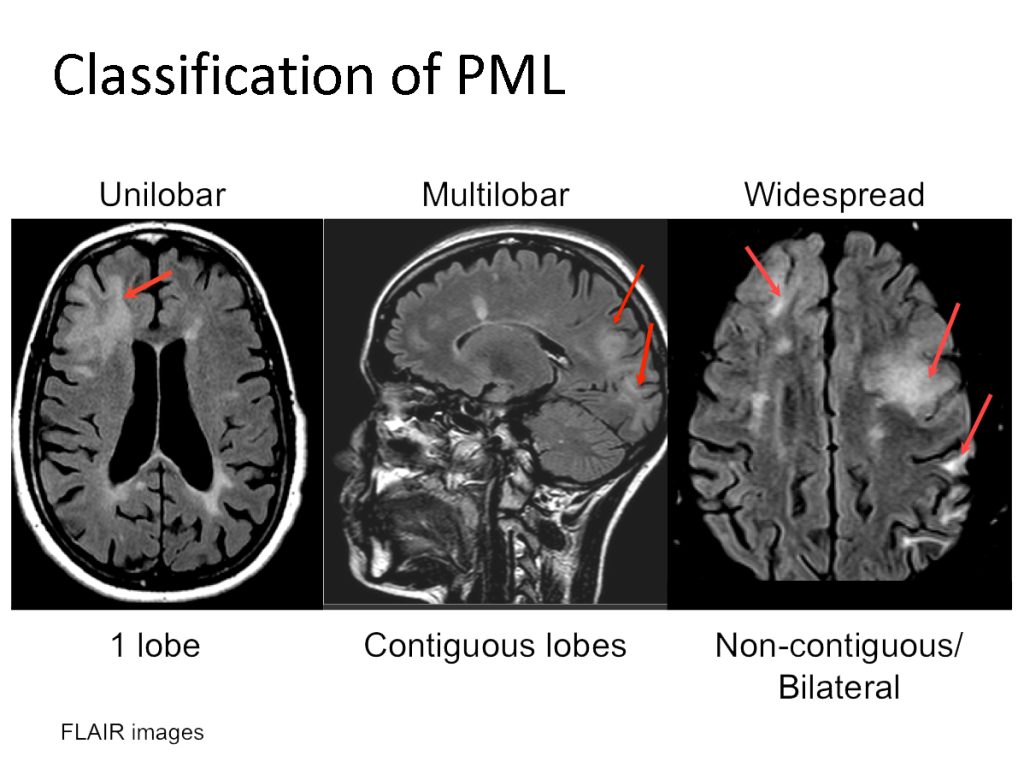
PML appears as a hyperintense lesion on T2 and FLAIR, hypointense on T1, and positive on DWI. Usually, there is no mass effect. Punctate or scattered faint gadolinium enhancement can occur in 50% of cases. MRI FLAIR is the most sensitive modality even in the posterior fossa. Lesions tend to be larger than MS plaques, located peripherally in the subcortical U-fibers with sharp borders towards the cortex and blurred towards the white matter. Punctate T1 enhancing or FLAIR hyperintense lesions with or without a central lesion is pathognomonic as is a starry night/ground glass appearance on T2 or FLAIR crescent lesions in the cerebellum. The frontal lobe followed by the parietal lobes tend to be the preferred sites but any area including the basal ganglia, capsule and posterior fossa can be involved.




The risk is influenced by JC virus serology index value, duration of treatment and prior immunosuppression therapy.
JC virus serology is an indirect measure of anti JC virus antibody titer. The index value is associated with a given level of forward looking risk for developing PML in the upcoming year. Negative patients are defined as having an index value of less than 0.2. Patients with an index value of 0.9 or less remain at a low risk of less than 1/1000, even after 5 years of exposure, while those with an index value of 1.5 or greater have a risk of 10/1000, or 1% per year, at that time.
The algorithm, though useful, has certain limitations. Approximately 10% of patients in our experience, with low index values, will flip between positive and negative. There is a reported 2.7% (95% confidence interval 0.7 -6.0 %) incidence of false negatives. There is a 2 % annual rate of seroconversion which, unlike the flippers, is accompanied by a significant and sustained elevation in the JC virus index value. Major and Frohman have reported a much higher rate of false negatives of approximately 35% though on a small sample (17 patients), based on positive viremia in patients with negative serology. Recently, there have been 3 reported cases of PML in patients with negative serology with one having had the test done 2 weeks prior to the onset of symptoms. The latter raises the question whether, in practical terms and for a given time point, we can determine with sufficient certainty if a patient is truly not a carrier of JC virus and thus perhaps should ignore the negative antibody status box in the algorithm and include the negative patients in the less than 0.9 category.
Borchardt et al in a post marketing study published in Multiple Sclerosis suggested that Biogen’s risk stratification underestimated the true risk. They determined that JCV-seropositive patients with prior immunosuppressant (IS) use, had an incidence of PML during months 25-48 of natalizumab therapy of about 19.5 per thousand. Those without prior IS use, the incidence during months 25-48 was approximately 7.4 per thousand, and during months 49-72, it was approximately 10.8 per thousand. If they limited the analysis to patients with a JCV index is in the range 0.9-1.5, then the incidence during months 49-72 was around 6.2 per thousand in comparison to 17.0 per thousand when the JCV index exceeded 1.5.
Prior immunosuppression use category in the algorithm is limited to older agents such mitoxantrone, methotrexate, azathioprine and cyclophosphamide. The newer therapies such as fingolimod, teriflunamide, dimethyl fumarate, alemtuzumab, ocrelizumab all immunosuppress to a variable degree, a factor that has not been taken into account in the elaboration of the latest version of the PML risk algorithm for natalizumab treated patients.
Low levels of L-selectin (CD62L) on CD4+ T cells in natalizumab treated patients has been shown to be predictive of PML. This finding however has not been confirmed by others.

Early detection is the first objective. PML can have a prolonged subclinical phase of up to 8 months in some cases. Diagnosis of PML in asymptomatic MS natalaizumab-treated patients has been associated with a mortality rate of near zero and an incidence of residual PML morbity of 30%.
The only proven therapy is immune reconstitution such as HARRT in HIV or stopping immunosuppression in transplants. In MS patients, it requires the removal of the immunosuppressing agent.
Natalizumab has a serum half-life of 11+/- 4 days but, after 4 weeks, alpha 4 integrin receptor saturation remain greater than 70%, thus maintaining clinical efficacy.
Khatri, Neurology. Feb 2009; 72(5) proposed doing 3 sessions (Q2days) of PLEX removing 1.5 plasma volumes per session starting at 2 weeks after the last infusion. The PLEX reduced plasma natalizumab concentrations relative to same patients without PLEX by 75% but did not, on average, change the receptor saturation levels.
He concluded by proposing a PLEX protocol, starting 1 week post last infusion, consisting of 5 sessions, 2 days apart, removing 1.5 plasma volumes which should result in a reduction in the alpha 4 integrin receptor saturation level
Rapid reintroduction of immune surveillance by removing natalizumab through PLEX is however associated with early and, at times, severe immune reconstitution inflammatory syndrome (IRIS). There are case reports of successfully treating natalizumab associated PML, especially those involving the posterior fossa, without using PLEX.
A recent study by Landi et al published in Neurology (Feb. 2017) reviewed 219 cases of natalizumab-PML and was unable to detect any benefit in survival or clinical outcome from PLEX. Country of origin and the develppment of PML-IRIS were the only predictors of mortality or poor outcome.
IRIS is mainly CD8+ T cell driven and occurs in all cases of PML in MS. It usually presents with neurological deterioration accompanied by enlargement of and/or new enhancement of the PML lesion with/out mass effect on MRI. It occurs days to weeks after PML treatment and can persists for several months. Early and aggressive treatment of IRIS with corticosteroids or azathioprine has been reported in a few cases to have encouraged the persistence and progression of PML. Some have suggested that the treatment of IRIS should therefore be instituted only when there is evidence of clinical deterioration. In an MS patient with PML whom natalizumab has been removed via PLEX the clinical deterioration can however occur for different reasons; PML progression, PML-IRIS development, MS rebound or a combination of the three. It may be difficult to determine either radiologically and/or clinically which of these or combination thereof is responsible.
Maraviroc, a chemokine CCR5 antagonist, can enhance CD4+ T cell proliferation and reduce T cell trafficking. It is unknown whether it can directly affect the JC virus. . Maraviroc 300mg BID has been used successfully in MS PML patients to treat IRIS in lieu of corticosteroids.
Enhancing the immune response using IL-2 or IL-7 with or without a JCV vaccine has been used with some success in a few cases of non MS related PML. IRIS could be a serious concern in MS related PML.
Passive immunization strategies such as JCV VP-1 specific monoclonal antibodies or JCV specific cytotoxic T lymphocytes are being researched. A similar available approach could be IVIG.
There is evidence that interferon gamma is involved in the regulation of JCV gene expression and its administration could inhibit JCV replication. In MS related PML we risk exacerbating the MS rebound.
Previous studies have failed to show any benefit from gancyclovir, leflunamide topotecan, mefloquine, cidofovir, ara-c and interferon alpha.
There are case reports claiming benefit from using mirtazepine or risperdone or other serotonin receptor inhibitors which theoretically could antagonize JCV cell entry.
Early detection is primordial
The decision to PLEX will depend on the location and size of the PML lesion with consideration of the potential IRIS related subsequent impact.
Mirtazepine and IVIG are safe and potentially helpful
Immunosuppressant therapy for IRIS or MS rebound either with maraviroc or corticosteroids should be dictated by clinical evolution, gadolinium enhanced MRI changes and CSF JCV titers.
DMF in clinical trials induced a 32% reduction from baseline in mean lymphocyte counts by week 8. The nadir tended to be achieved by month 6. On average, approximately 5% of patients reached a grade 3 or more lymphocytopenia.
Cases of PML have been reported with all three commercially available fumaric acid esters. Cases were associated with older age and persistent grade 3 lymphocytopenia
More recently, however, 3 cases of PML unassociated with persistent grade 3 lymphocytopenia have been noted.
In pivotal studies, fingolimod reduced ALC to a mean of 500 cells/mm3 and was discontinued only when ALC fell below 200 cells/mm. In one series, 15% of patients reached grade 4 (< 200 /mm) lymphocytopenia. Only a few cases of fingolimod associated PML have been reported.
The FDA has issued a warning on the fingolimod label but as of recent no recommendations have been issued.